Research Projects
HIGHLANDER

The objective of HIGHLANDER is to develop membrane electrode assemblies (MEAs) for Heavy-Duty Vehicles (HDV) with disruptive, novel components, targeting stack cost and size, durability, and fuel efficiency. The project will design, fabricate, and validate the HDV MEAs at cell and short stack level against heavy-duty relevant accelerated stress test and load profile test protocols. The unique approach of HIGHLANDER is to develop core fuel cell components in tandem, ensuring their greatest compatibility and lowest interface resistance: ionomer and reinforcement, catalyst and catalyst support, catalyst layer composition and property gradient in tune with the bipolar plate flow-field geometry. Materials screening efforts will be supported by the development and use of improved predictive degradation models bridging scales from reaction sites to cell level. Model parameterisation is implemented using experimental characterisation data at materials, component, and cell level. HIGHLANDER brings together a European supply chain of fuel cell materials and components producers and an OEM stack developer that makes this approach possible. HIGHLANDER aims to bring about a significant reduction in stack cost and fuel consumption through improvement of catalyst coated membrane performance and development of a new, lower cost single-layer gas diffusion layer. It will aim to achieve the 1.2 W/cm² at 0.65 V performance target at 0.3 g Pt/kW or below, meeting a lifetime target of 20,000 h. Sustainability considerations include benchmarking of fluorine-free membranes for HDV MEA application and re-use of platinum in the context of a circular economy.
Website: https://highlander-fuelcell.eu/
This project has received funding from the Clean Hydrogen Partnership under grant agreement No 101101346. This Joint Undertaking receives support from the European Union's Horizon 2020 Research and Innovation program, Hydrogen Europe and Hydrogen Europe Research.
H2Meer
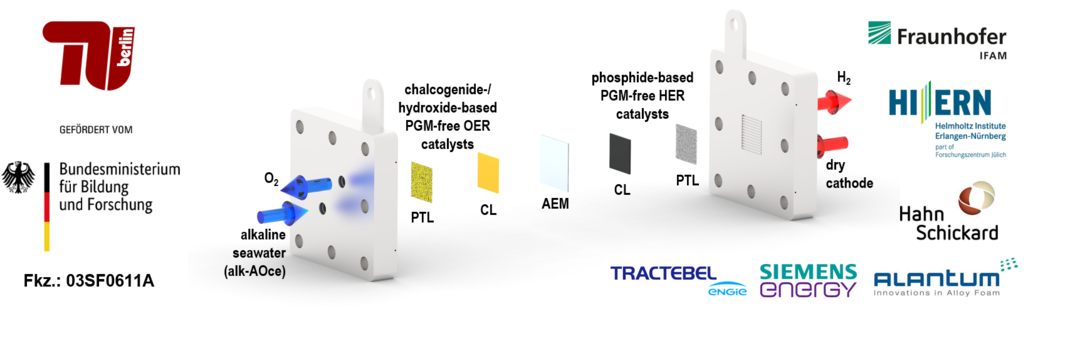
In the BMBF-funded project H2Meer, advanced seawater electrolyzer cell designs and operation modes are developed in order to achieve industrially relevant performances for low-cost green hydrogen (H2) production in (hyper-)arid regions. The main challenges for a sustained direct electrolytic splitting of seawater lie in the control of unfavorable precipitation of poorly soluble metal hydroxides and carbonates, as well as the identification of selective, corrosion-resistant materials. Despite showing high catalytic activities, state-of-the-art platinum group metals (PGMs) undergo dissolution from the electrodes over time, which can be attributed to the presence of chloride anions in saline electrolytes. In H2Meer, entirely noble-metal-free cell components - featuring novel nanostructured catalyst materials and porous transport layers (PTLs), as well as customized anion-exchange membranes (AEMs) and ionomers - are synthesized. At the anode, highly-selective OER electrocatalysts are utilized for a minimization of the undesirable formation of toxic chlorine (Cl2) gas or hypochlorite (OCl-) species.
Nanofocused X-ray Probes for the Elucidation of Structure-Property-Selectivity Relations of Cu-based eCO2RR Catalysts
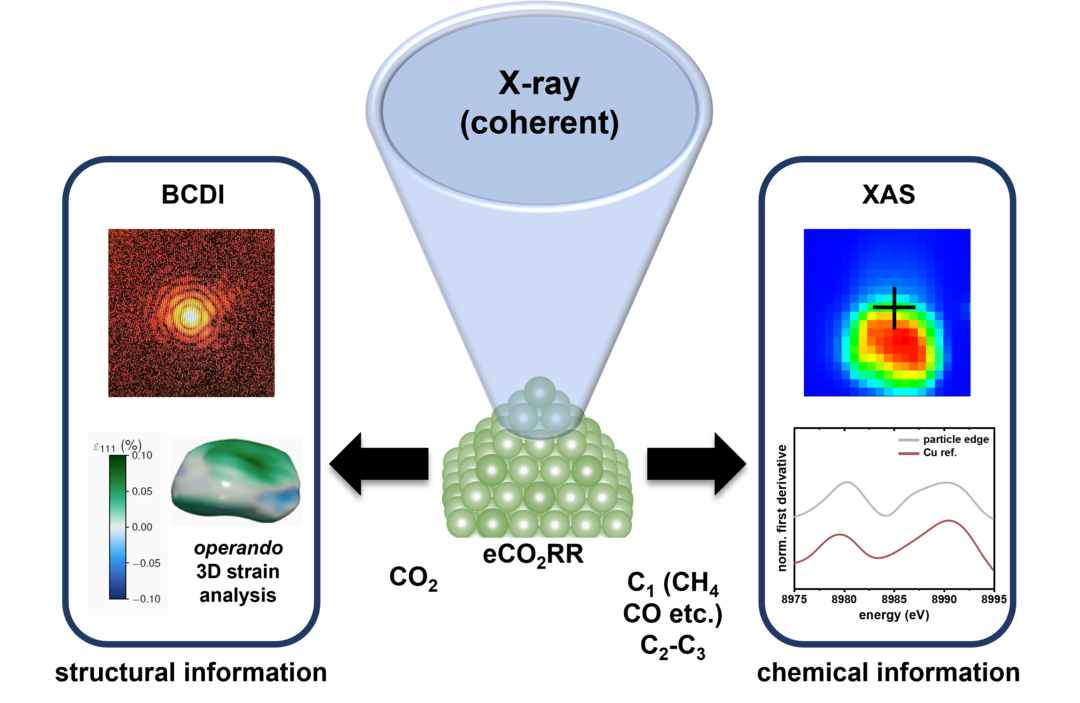
Advanced operando X-ray techniques can provide distinct insights into various electrocatalytic processes. In the context of the electrochemical CO2 reduction (eCO2RR), controlling the product selectivity still remains a major challenge. In this project funded by the Alexander von Humboldt Foundation (AvH), a novel combined X-ray absorption (XAS) and coherent diffraction (BCDI) approach is developed. Ultimately, the identification of active sites of mono- and bimetallic copper-based nanoparticles in eCO2RR is pursued.
DaCapo
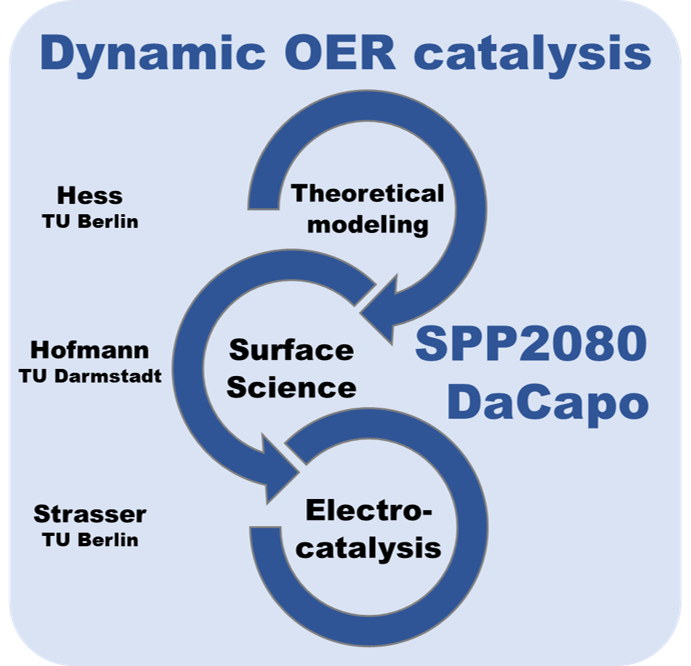
DaCapo: Dynamically driven rutile-based electrocatalysts for acidic oxygen evolution beyond stationary efficiency
The DaCapo project is a part of the DFG-funded Priority Programm SPP 2080 “Catalysts and reactors under dynamic conditions for energy storage and conversion“.
With the DaCapo project, we aim at contributing to the fundamental understanding of dynamic operation of electrocatalytic systems and to explore the extent to which an electrochemical reaction, that is the relevant O2 evolution reaction, can be controlled and possibly enhanced via dynamic resonance theory. We will form a bridge between atomic insights into the dynamics of active site and active surface phases of well-defined catalytic model surfaces and real catalyst materials and multiscale simulations of their dynamic behavior, exemplified by rutile based OER catalysts (IrO2, RuO2, mixed (Ru-Ir-Ti)O2) under the influence of forced oscillatory operation. This bridge will be achieved by combining UHV and in situ/operando surface science, electrochemical methods, and theoretical modelling.
H2Mare
H2Mare ist eines der drei großen Wasserstoff-Leitprojekte des Bundesministeriums für Bildung und Forschung (BMBF). Dabei untersuchen wir die direkte Meerwasserelektrolyse für eine simple und alternative Möglichkeit Wasserstoff offshore (bzw. nearshore) herzustellen. Bei der dafür notwendigen Katalysatorentwicklung wird der Fokus auf edelmetallfreie Anodenmaterialien gelegt um diese im pH neutralem Meerwasser einsetzen zu können. Die verschiedenen im Meerwasser vorhandenen Salze stellen dabei eine große Herausforderung dar, welche innovative Herangehensweisen benötigt.
Durch die Zusammenarbeit mit dem DVGW-EBI werden außerdem Fouling-Einflüsse sowie die Umweltverträglichkeit der eingesetzten Materialien untersucht. Als weiterer Projektpartner entwickelt das HZ Hereon Mikro- und Ultrafiltrationseinheiten um Schwebstoffe und Partikel aus dem Meerwasser zu entfernen und so die Robustheit des Prozesses zu verbessern.

DACStorE

The latest report by the Intergovernmental Panel on Climate Change has shown that merely reducing CO2 emissions is no longer enough to achieve the two-degree target. We must also capture emissions that have already been produced and store them in the long term. CO2 capture from the air and its storage represents an important technical solution for reducing CO2 in the atmosphere in the early stages of development.
The aim of the research project DACStorE (A Comprehensive Approach to Harnessing the Innovation Potential of Direct Air Capture and Storage for Reaching CO2-Neutrality) is to prepare a sustainable market ramp-up of DACS technology. Six Helmholtz centers, in cooperation with the Technical University of Berlin, are researching technical solutions for CO2 capture from the air and its storage in geological formations. The focus is on three technical approaches to CO2 capture, which are being researched, compared and further developed to prototypes in an Open Technology Approach. A major focus is on reducing energy requirements and on scale-up opportunities by developing and testing suitable materials, designs and operational concepts. Requirements that large-scale industrial production imposes are also addressed in the early development phase. In addition to the technical properties, the economic and ecological aspects of the technologies investigated, their acceptance in society and legal framework conditions are examined. The aim is to identify coordinated technology options and operating conditions for different locations. Furthermore, important requirements are to be pointed out to the technology developers in an early phase of development in order to enable a successful market introduction and market ramp-up.
https://www.fz-juelich.de/de/iek/iek-3/projekte/dacstore
This project has received funding from the Initiative and Networking Fund of the Helmholtz Associa-tion (grant agreement No. KA2-HSC-12, ‘A Comprehensive Approach to Harnessing the Innovation Potential of Direct Air Capture and Storage for Reaching CO2 -Neutrality’, DACStorE).
ANEMEL

ANEMEL (ANion Exchange Membrane Electrolysis from Low-grade water sources): will gather expertise in the field of electrocatalysts, membranes and electrolysers – the overall goal is a prototype that yields green hydrogen from low-grade water with minimal treatment. Our team at TUB is workpackage leader within the workpackage about MEA single cell measurements.
The ANEMEL project is led by the National University of Ireland Galway (NUIG) (Ireland), and counts on the participation of Technical University of Berlin (TUB) (Germany), AGFA (Belgium), LEITAT (Spain), AGATA Comunicación Científica (Spain), De Nora (Italy), Technion Institute of Technology (Israel), The University of Newcastle upon Tyne (U.K.), École Polytechnique Fédérale de Lausanne (EPFL) (Switzerland) and Haute École Spécialisée de Suisse Occidentale (HES·SO) (Switzerland).
This project has received funding from the European Union’s Horizon Europe research and innovation programme under Grant Agreement No. 101071111. The project is part of the Green Hydrogen Challenge portfolio of the European Innovation Council (EIC) programme.
Website: https://anemel.eu/
BRAVA
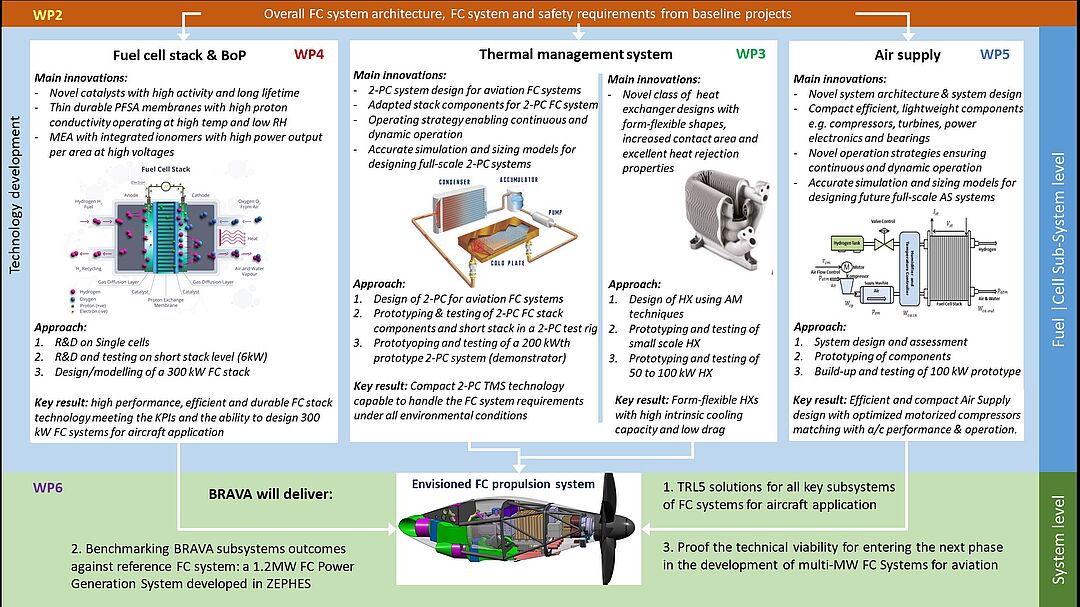
The Proton Exchange Membrane Fuel Cell (PEMFC) technology emerging from the automotive industry is of high interest for the future aeronautic industry. While automotive industry fuel cell (FC) systems are usually limited to 100 kW, aircrafts require a significantly greater amount of power (multi-MW) and must operate at different temperatures and pressures while maintaining the system's safety and reliability levels according to aeronautical standards.
In the BRAVA project, we are working on novel catalyst/support systems with a focus on high durability and optimized dispersion of particles at high platinum loadings. The intermetallic catalysts are synthesized through scalable wet impregnation synthesis with consecutive heat treatment and characterized for their electrochemical performance, as well as their physicochemical properties.
In cooperation with our partners, we integrate our catalysts in the fuel cell stack to perform under harsh conditions for aviation.
Website: https://brava-project.eu/
“Funded by the European Union under grant number 10110149. Views and opinions expressed are however those of the author(s) only and do not necessarily reflect those of the European Union. Neither the European Union nor the Clean Hydrogen Joint Undertaking can be held responsible for them.”

EcoFuel
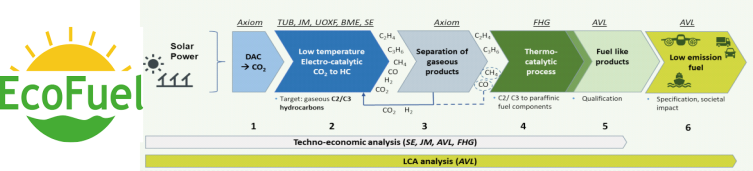
EU-funded EcoFuel project that will create and demonstrate an innovative process chain able to improve the energy efficiency for production of synthetic fuel from CO2 and water using renewable energy. This next-generation renewable fuel for energy and transport will increase energy efficiency and reduce costs associated with energy conversion from CO2.
The rising share of solar- and wind-based renewable electricity worldwide beyond the current capacity of the electrical grids generates serious large-scale electrical energy storage needs. To remedy this electricity storage challenge, scalable “Power-to-X” technologies are needed that enable the coupling and integration of different sectors, such as electricity, heat, mobility, and industrial production. The coupling of renewable electricity and mobility is of particular importance. Electromobility concepts for light-duty vehicles and applications allow for significant reductions of CO2 emissions thanks to direct electricity storage in battery vehicles. However, several important transport modes, such as aviation, maritime shipping, long-distance heavy-duty transport will remain critically dependent on high energy-density, hydrocarbon-based liquid fuels in the future. In order to cut carbon dioxide emissions also in these transport sectors and, at the same time, comply with the new EU RED II directive, admixtures of synthetic carbon-neutral liquid hydrocarbon fuels will be indispensable. This is why the efficient and sustainable production of synthetic, carbon-neutral, high energy density liquid hydrocarbon fuels from renewable electricity, water and CO2 has become a technology development priority.
The EcoFuel project concept centers on the integration of a set of chemical process steps into a complete process chain for the generation of renewable, high-energy density liquid hydrocarbon fuels for mobility applications. The process steps include feed supply, purification, as well as electro-catalytic and thermo-catalytic conversion processes.
The individual EcoFuel process steps involve the electro dialysis-based concentration of atmospheric CO2, which, together with water and renewable electricity, is electrochemically converted into olefinic and paraffinic gaseous hydrocarbon molecules (such as ethylene, ethane, propylene, propane, acetylene, and possibly others), followed by their separation and subsequent thermo-chemical reactive conversion into renewable liquid C6+ hydrocarbon fuels suitable for use in combustion engines.
https://www.ecofuel-horizon.eu/
This project has received funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement No 101006701.
ReAlCharge
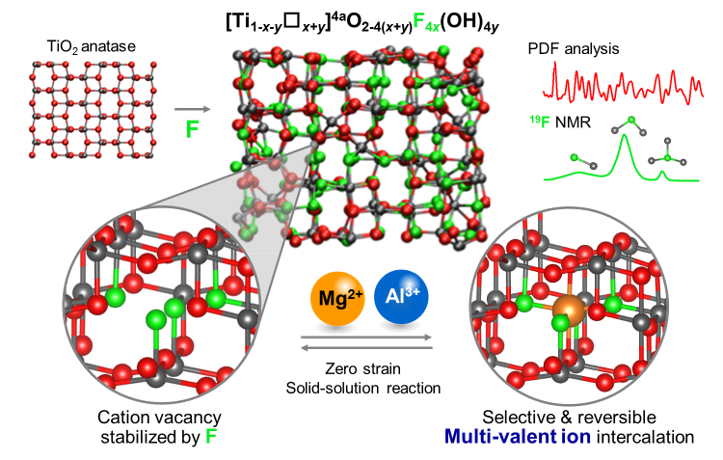
To support the growing demand for electrochemical energy storage, complementary or even superior technological solutions over traditional Li-ion batteries are urgently needed. Aluminum batteries are particularly attractive candidates owing to their high projected volumetric energy density, low cost, high safety. Moreover, being the most abundant metal on the Earth crust, aluminum also matches the sustainability requirement that is mandatory to develop durable technologies. A critical feature of Al3+ intercalation chemistry is the high charge density of this cation that ultimately affects the electrolyte properties and the ability of host frameworks to reversibly accommodate a large proportion of Al3+ thus generating high energy. In this German-French consortium project, we study fundamental aspects as well as more applied cell-related challenges of the interfacial processes and materials chemistry pertinent to high energy density aluminum batteries. We achieve this by working on a range of cell components and cell processes simultaneously, such as the electrolytes, electrode materials and electrode-electrolyte interfaces. We are focusing on oxide-based electrode materials with controlled amounts of cationic vacancies, that were shown in our earlier work to enable solid-state diffusion of Al3+ while providing additional insertion sites. Concomitantly, physicochemical properties of suitable electrolytes and their interface with Al and the positive electrode material are characterized using a wide array of in-situ und ex-situ techniques, such as X-ray Absorption, Nuclear Magnetic Resonance, high resolution Transmission electron microscopy, X-ray scattering and pair distribution function and others. Specifically, exploiting our defect engineering approach, the French group at SORBONNE UNIVERSITÉ (Leader: Associate Prof. Damien Dambournet) is designing novel intercalation compounds consisting of oxidic networks with unprecedented large content of cationic vacancies. We at TU Berlin are studying the electrochemical dynamics of Al3+ intercalation of the new materials designed by the French group.


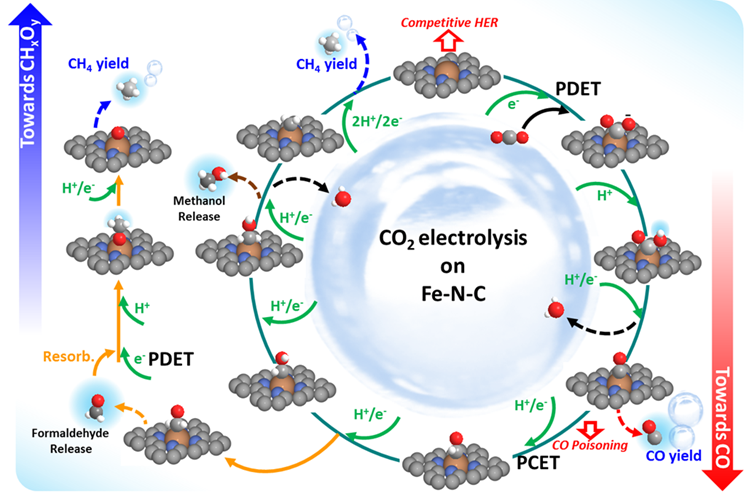
SELECTCO2

SELECTCO2 aims to contribute to the electrification of the chemicals industry through the development of highly selective and efficient devices for the conversion of CO2 to high value products at low temperatures and pressures. It is well known in the chemicals industry that costs due to separations can amount to 60-80% of the total costs of the chemicals. This same general cost percentage holds in bio-based chemicals as well Electrochemical CO2 Reduction (ECO2R) allows the unique ability to start with a single reactant in CO2 and use catalysis to build up selectively to a given molecule. Direct conversion to a specific product allows for the mitigation or even elimination of separation costs and can greatly reduce the costs of producing a given chemical.
https://www.selectco2.eu/index.php/en/
The research leading to these results has received funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement SELECTCO2 No. 851441.
CO2 Reduction Reaction
CRESCENDO-Non noble metal containing MNC catalysts for fuel cells
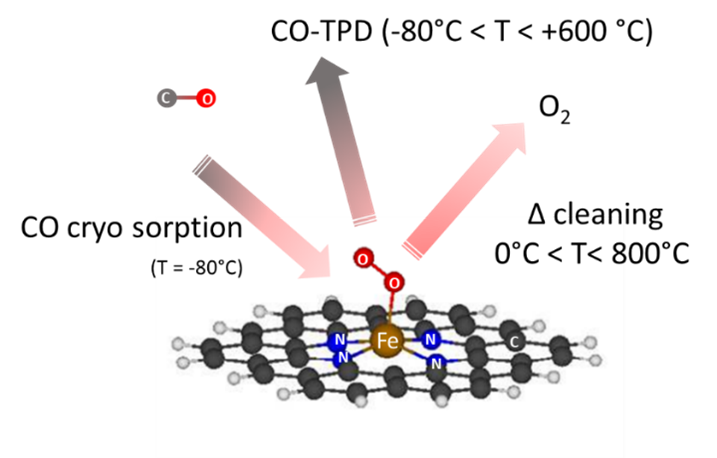
Metal nitrogen carbon catalysts (MNC) are a promising alternative for expensive and scarce platinum based catalysts in the cathodic oxygen reduction reaction in PEM fuel cells. The materials used are carbon based with integrated active Metal-Nitrogen centers. In our research, the improvement of this catalysts in terms of activity and stability is our main focus. We developed a new method to quantify the active site density and the derived turnover frequencies with a selective cryo CO-chemisorption method. This allows a targeted improvement of our newly developed catalysts. In collaboration with our international collaborators in the EU financed project CRESCENDO we currently work on further development of the catalyst, a better understanding about the materials, upscaling the synthesis as well as the implementation of MNC catalysts in a working fuel cell stack for automotive use.

Literature:
1. Luo, F.; Choi, C. H.; Primbs, M. J. M.; Ju, W.; Li, S.; Leonard, N. D.; Thomas, A.; Jaouen, F.; Strasser, P., Accurate Evaluation of Active-Site Density (SD) and Turnover Frequency (TOF) of PGM-Free Metal–Nitrogen-Doped Carbon (MNC) Electrocatalysts using CO Cryo Adsorption. ACS Catalysis 2019,9 (6), 4841-4852.
Industry funded project
In this industry funded project, we modify the ORR catalysts´ support to gain a closer insight into the catalyst layer during MEA operation. N-modified high surface area carbons are promising candidates revealing increased performance and enhanced stability. The interaction between the ionomer film and the catalyst itself is of special focus within this project and can be analyzed using different applying different electrochemical testing tool and settings.
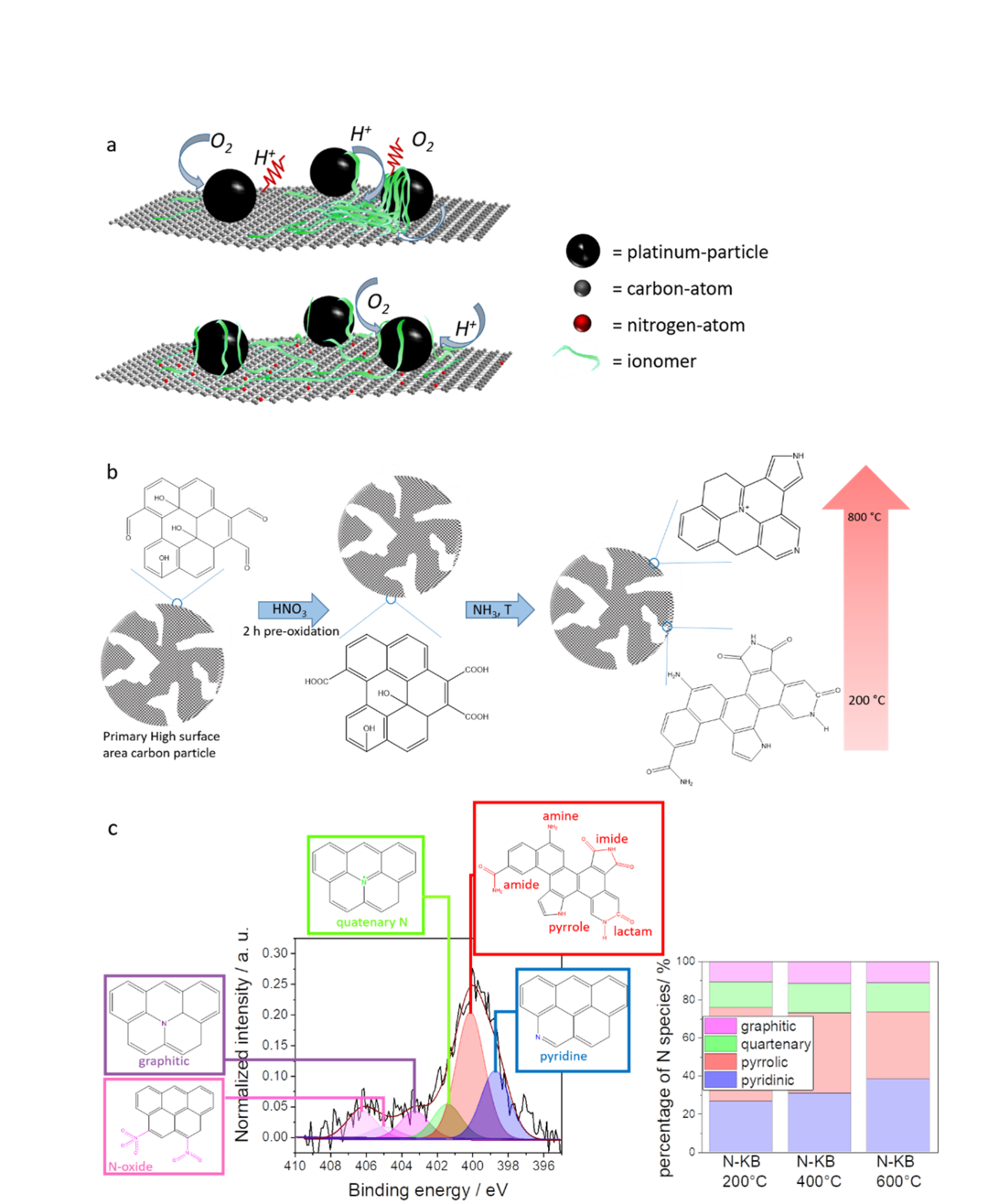

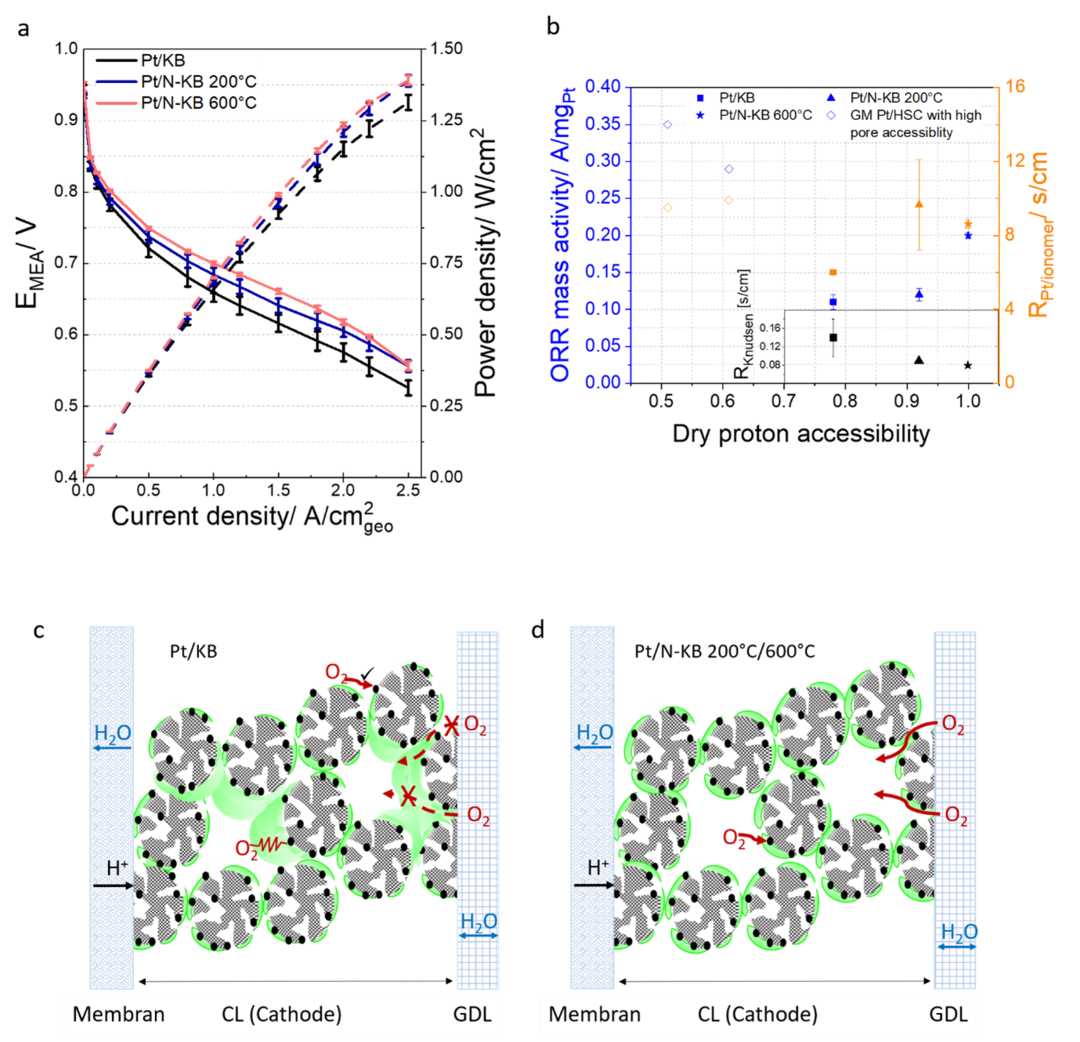
KorrZellKat: Corrosion Resistant Catalysts and Supports for Low-Temperature PEM Fuel Cell Cathodes
The bilateral german-israeli cooperation project „KorrZellKat“ develops new high-performance materials for the solution of the two most urgent challenges of the low temperature proton exchange membrane fuel cell (PEMFC) namely the cost and the durability. State-of-the-art catalyst/support techniques for the catalytic reduction of molecular oxygen to water at the PEMFC cathode are carbon-based supported Pt nanoparticles with high metal loading. But the carbon support material is not stable under the oxidative operating conditions of the PEMFC and limit the duration. The high noble metal loading increased the cost.
The overall aim of the Federal Ministry of Education and Research-funded project is on one side the development and understanding of strongly corrosion-resistant support material for Pt-based catalyst. Additionally the developed corrosion-resistant carbon materials act as noble metal-free catalysts. Such Pt-free catalysts can be implemented directly as PEMFC cathode material or be used as supports for non-noble metal catalysts.
GAIA
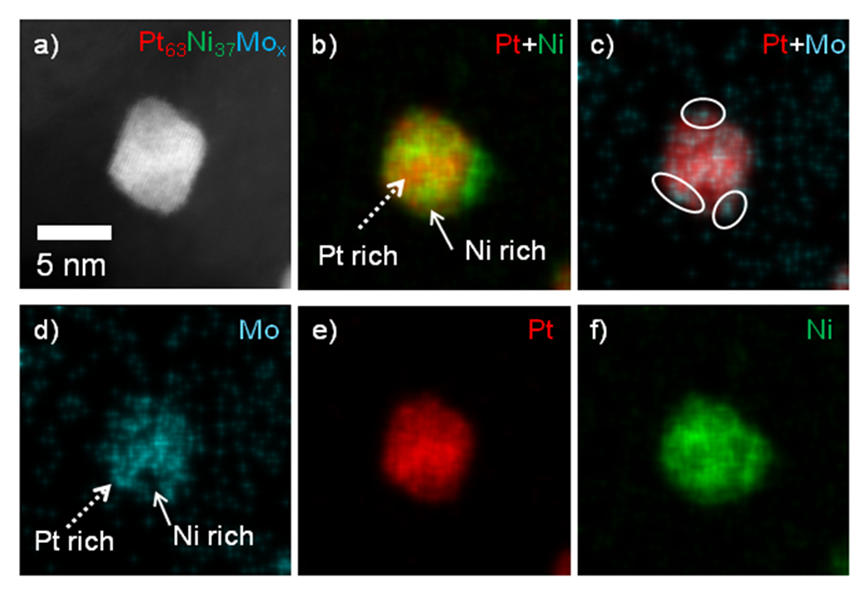
GAIA aims to developing a high performance automotive MEA that provides the materials and designs that satisfy the cost target by providing high power density at high current density, while also attaining the other essential objectives of durability, reliability and high operation temperature. Its intention is to:
- Realise the potential of these components in next generation MEAs showing a step-change in performance that will largely surpass the state of the art by delivering a beginning of life power density of 1.8 W/cm2 at 0.6 V;
- Validate the MEA performance and durability in full size cell short stacks, with durability tests of 1,000 h with extrapolation to 6,000 h
- Provide a cost assessment study that demonstrates that the MEAs can achieve the cost target of 6 €/kW for an annual production rate of 1 million square metres.
GAIA will develop and bring together advanced critical PEM fuel cell components. These components will be integrated into a fuel cell that is capable of delivering on the most challenging of the performance, cost and durability targets required for large-scale automotive fuel cell commercialisation. New designs, architectures, constructions and deposition methods will be used at the level of the components and at the level of their integration in the catalyst layer, where novel constructions and coating methods will be used. While some of the materials and components are early developments with the technology concept formulated, the majority have already been proven experimentally. These components will be further advanced, optimised and integrated into next generation automotive MEAs to demonstrate the application of the technology.
The GAIA project has received funding from the Fuel Cells and Hydrogen 2 Joint Undertaking (now Clean Hydrogen Partnership) under grant agreement No 826097. This Joint Undertaking receives support from the European Union’s Horizon 2020 research and innovation programme, Hydrogen Europe and Hydrogen Europe Research.”


Hydrogen peroxide production
Hydrogen peroxide (H2O2) is commonly produced via the anthraquinone process, but it still suffers from high energy consumption and the generation of a variety of by-products. A promising alternative is the electrochemical pathway. We present the micro flow cell as a suitable method for the production of hydrogen peroxide via two-electron oxygen reduction using benchmark carbon gas diffusion layers. Inspired by the rotating ring disk electrode (RRDE) method an expanded micro flow cell was introduced, using a build in second working electrode for the oxidation of the produced hydrogen peroxide. Converting the resulting current at a constant potential into the produced amount of hydrogen peroxide, allows the direct sensoring of the catalytic activity.
Direct seawater electrolysis
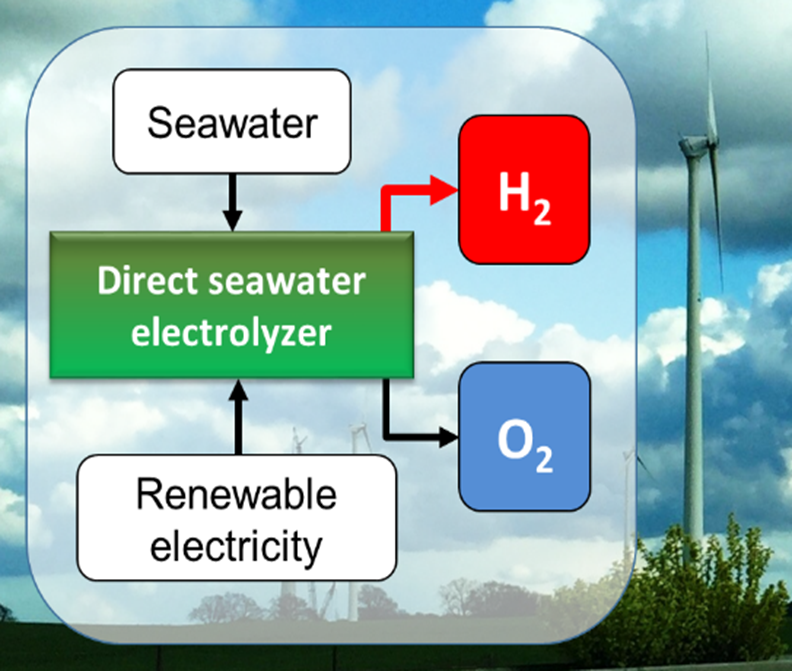
This project investigates the electrochemical and selective splitting of seawater into hydrogen and oxygen. Especially in seawater containing chloride causes side reactions at the anode, in which chloride oxidizes into toxic chlorine gas or hypochlorite. Thus, the direct seawater electrolyses seeks for new material that surpress the side reaction to make the direct utilization of seawater for the production of hydrogen feasible.
